


Since 1 May 2007, registration in the national Database of Medical Devices (BD/RDM) met the procedures established by the Ministerial Decree of 21 December 2009.
| Date of placement on the market | Type of Medical Devices (MD) | Registered address of the Manufacturer/Authorised representative | Registration in the Database |
|---|---|---|---|
| Before 1 May 2007 | MDs of Class I, IIA, IIB, III and active implantable devices | Any | Optional if the device has not been modified since 1 May 2007 with changes that require notification |
| After 1 May 2007 | MDs of Class I and assembled devices | EU country other than Italy | Optional |
| Italy | Mandatory | ||
| MDs of Class IIA, IIB, III and active implantable devices | Any | Mandatory |
The Database (BD/RDM), including the subset of database called “Repertorio”, constitutes a collection of information provided by:
Whoever makes the registration assumes full responsibility for the information provided, regarding both general data and data relating to each medical devices (MDs).
The publication of the data does not constitute any form of approval by the Ministry.
The Directorate General of Medical Devices and Pharmaceutical Service has the right to carry out checks at any time on declarations made and on registered medical devices, as established by Legislative Decree No 46 of 24 February 1997 and subsequent amendments.
In the Database of Medical Devices, entities who enter the data directly are considered “registrants”. Only registrants can change the information relating to medical devices that they have registered.
All information, initially acquired by the system in processing status “L”, must be “validated” by electronic signature in order to be “published”.
Since 1 January 2014, with regard to the processing status (“L”), there has been a time limit in force for the completion of data notifications. The system applies a different time limit in relation to the “Processing Start Date”:
As well as in the Database, devices can also be registered in the subset of database called “Repertorio” or “Repertoire” in the User Manual Medical Device Manufacturer Profile. Registering a medical device in the “Repertorio” is not mandatory and it is free of charge.
Please note that the registration in the “Repertorio” gives the Regions and the Autonomous Provinces of Trento and Bolzano as well as the operators of the National Health Service full visibility of the information on the registered medical devices.
To do this, just click on “Register in the Repertoire”. This function can also be activated after the validation of the data by approved electronic signature.
In order to register medical devices in the BD/RDM, the Database must be accessed according to the two possible alternative paths defined below (A and B). The company that operates directly in the BD/RDM is defined as a “registrant company”; a registrant company can be:
Once access is allowed, company can enter data of the devices.
Before being able to proceed with the registration of medical devices, you must access the Database of Medical Devices according to the instructions given in the previous section “Accessing the BD/RDM”.
About procedures in order to register medical devices, please see the instructions in the User Manual Medical Device Manufacturer Profile. We remind you that the registration of medical device is mandatory or optional depending on the risk class of the medical devices and the registered address of the parties responsible for placing them on the market. For further information, please see Requirements for registration.
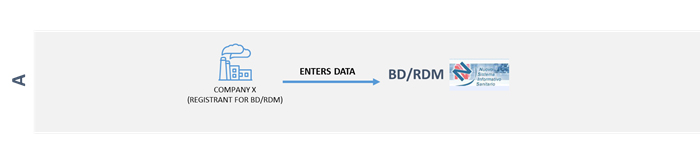
Your company, as a manufacturer, authorised representative, assembler wants to access directly and therefore be considered a registrant in the BD/RDM.
If your company is registered in Italy: access the Database via impresainungiorno.gov.it

The Database is accessed via the online Medical Devices service at impresainungiorno.gov.it under Servizi integrati di impresa.gov - La mia scrivania.
The online service can be accessed via SPID (Public Digital Identity System), CIE (Electronic Identity Card) or CNS (National Services Card).
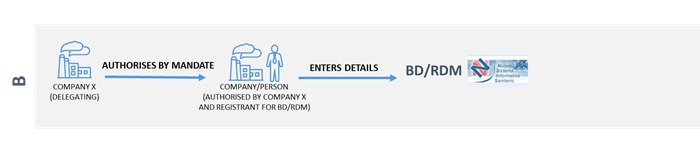
The legal representatives of the companies concerned are directly authorized to use the service; moreover, the legal representative of a company can authorise one or more trusted persons to operate on the online Medical Devices service.
To delegate, the legal representative must use the sub-delegation services available on the business portal under Servizi Integrati at impresainungiorno.gov.it - Gestione Subdeleghe.
At the end of this procedure,users will have access to the functions for managing medical devices for which they have been authorised.
For registering a medical device, the authorised person must have a qualified electronic signature issued by one of the approved certifiers in Italy or in compliance with the provisions of Regulation (EU) No 910 / 2014- The eIDAS (electronic IDentification Authentication and Signature). Instructions about entering data are provided in the User Manual Medical Device Manufacturer Profile.
If your company is not registered in Italy: access the Database by sending the login credentials request form via email.
Procedure for requesting login credentials is:
N.B. Even if your company is not a manufacturer, for example an authorised representative, the profile enabling device data entry is called “DM Manufacturer”.
For using the BD/RDM and registering a medical device, the person responsible for entering data in the BD/RDM must have a qualified Electronic Signature issued by an approved certification body in Italy or in compliance with the provisions of Regulation (EU) No 910/2014 - The eIDAS (electronic IDentification Authentication and Signature) Regulation.
Your company, as a manufacturer, authorised representative or assembler, wants to authorise another entity to access the BD/RDM, who is considered the registrant, responsible for entering data.
The authorisation can be made by a company (delegating company) to:
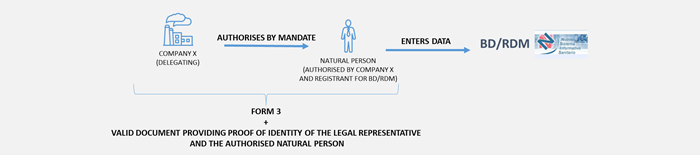
In the case of Italian authorised and registrant company - the authorisation procedure includes the following steps:
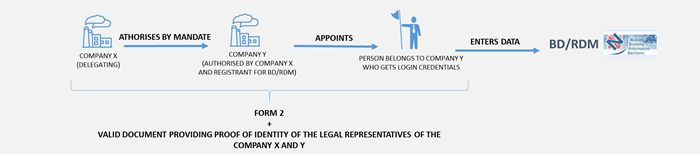
In the case of a foreign authorised and registrant company - the authorisation procedure includes the following steps:
N.B. Even if your company is not a manufacturer, for example an authorised representative, the profile enabling device data entry is called “DM Manufacturer”.
For using the BD/RDM and registering a medical device, the person responsible for entering data in the BD/RDM must have a qualified Electronic Signature issued by an approved certification body in Italy or in compliance with the provisions of Regulation (EU) No 910/2014 - The eIDAS (electronic IDentification Authentication and Signature) Regulation.
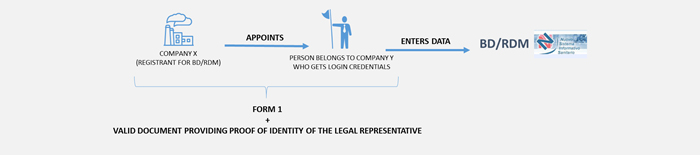
In the case of an authorised and registrant natural person - the authorisation procedure includes the following steps:
N.B. Even if as an authorised natural person does not perform the role of manufacturer as required by law, the profile enabling device data entry is called "DM Manufacturer".
For using the BD/RDM and registering a medical device, the person responsible for entering data in the BD/RDM must have a qualified Electronic Signature issued by an approved certification body in Italy or in compliance with the provisions of Regulation (EU) No 910/2014 - The eIDAS (electronic IDentification Authentication and Signature) Regulation.
Changes to data already entered in the system
The data already entered in the system can only be changed by the registrant who entered the data. For instructions on how to change medical device registration data, please see the instructions in the User Manual Medical Device Manufacturer Profile.
In the case of changes to data entered by another registrant, please contact the Ministry of Health Support Service: SupportoRDM - supportoRDM@sanita.fvg.it.
The support service involves IT/administrative checks which require variable time.
Support for users is divided into several specialist support services according to type of query:
For support and queries on accessing the system via impresainungiorno.gov.it, used by Italian companies, see website impresa.gov.it.
For questions and support regarding the technical rules of the procedure and how to register a device in the database and in the medical device subset of the database called “Repertorio” and how to change data already entered in the system, the Support Service of the Ministry of Health, SupportoRDM, due to the current health emergency and the change in working methods, from 2 November 2020, is available from Monday to Friday 9 am - 4 pm excluding holidays via e-mail at: supportoRDM@sanita.fvg.it.
For information on how to log in to the system, using the application and reporting malfunctions of the IT procedures, you can contact the new Service Desk of the Ministry of Health at the toll-free number 800 178 178 at the following times:
from Monday to Sunday (24h 7/7), including holidays.
You can also send reports to the following e-mail address: servicedesk.salute@smi-cons.it
Data di ultimo aggiornamento 13 gennaio 2023