Even exergonic, energy-releasing reactions require a small amount of activation energy in order to proceed. Endergonic reactions require much more energy input because their products have more free energy than their reactants. Within the cell, from where does energy to power such reactions come?
Biomolecules all carry energy in the form of their chemical bonds. However, rather than using this energy directly, cells break down glucose and other biomolecules in a controlled way, in order to produce adenosine triphosphate, or ATP. This is because the energy in ATP is easily accessible thanks to its structure.
ATP is a small, relatively simple molecule, but it contains the potential for a quick burst of energy that can be harnessed to perform cellular work. Think of this molecule as the cells’ primary energy currency in much the same way that money is the currency that people exchange for things they need. ATP powers the majority of energy-requiring cellular reactions.

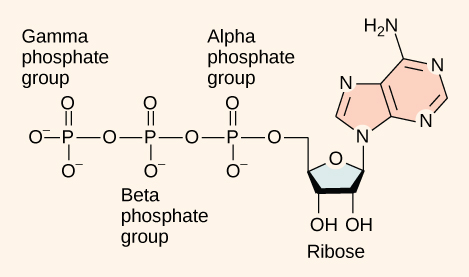
ATP is essentially an RNA nucleotide with two additional phosphate groups. Adenosine consists of the nitrogenous base adenine and a five-carbon sugar, ribose. Adenosine is bound to three phosphate groups. The bonds that link the phosphates are high-energy bonds that, when broken, release sufficient energy to power a variety of cellular reactions and processes. These bonds are “high-energy” because the products of such bond breaking—adenosine diphosphate (ADP) and one inorganic phosphate group (Pi)—have considerably lower free energy than the reactants: ATP and a water molecule. Because this reaction takes place using a water molecule, it is a hydrolysis reaction. In other words, ATP hydrolyzes into ADP in the following reaction:
ATP + H2O → ADP + Pi + free energy
Like most chemical reactions, ATP to ADP hydrolysis is reversible. The reverse reaction regenerates ATP from ADP + Pi. Since ATP hydrolysis releases energy, ATP regeneration must require an input of free energy.
ADP + Pi + free energy → ATP + H2O
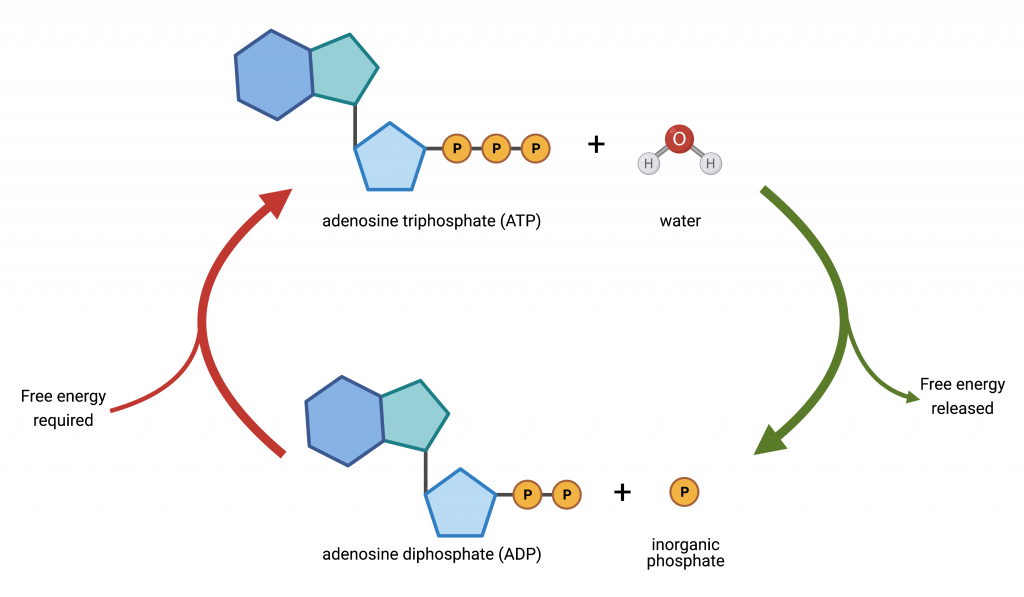
ATP is a highly unstable molecule. Unless quickly used to perform work, ATP spontaneously dissociates into ADP + Pi, and the free energy released during this process is lost as heat. The second question we posed above discusses how the energy released by ATP hydrolysis performs work inside the cell. This depends on a strategy that scientists call energy coupling . Cells couple the exergonic reaction of ATP hydrolysis to whatever work needs to be done.
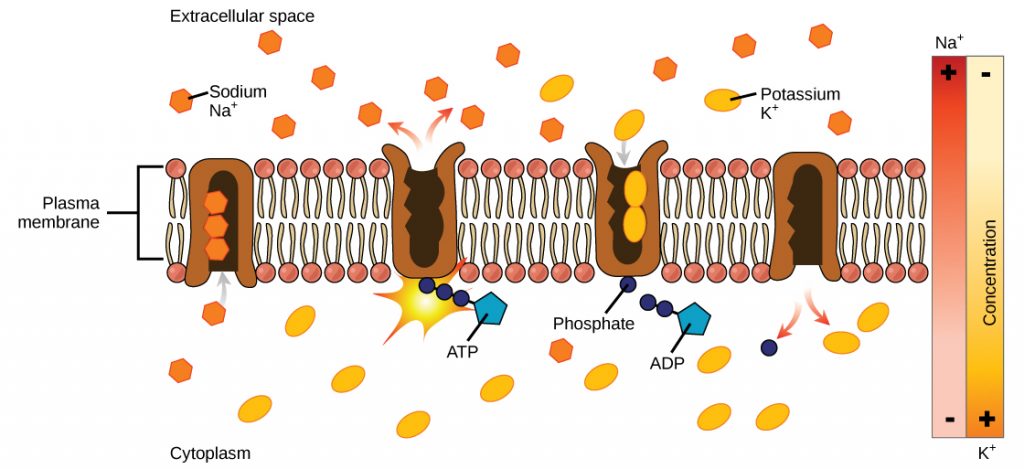
One example of energy coupling using ATP is the sodium-potassium pump (Na + /K + pump), which drives sodium out of the cell and potassium into the cell. A large percentage of a cell’s ATP powers this pump, because cellular processes bring considerable sodium into the cell and potassium out of it. The pump works constantly to stabilize cellular concentrations of sodium and potassium. In order for the pump to turn one cycle (exporting three Na+ ions and importing two K + ions), one ATP molecule must be hydrolyzed. When ATP hydrolyzes, its gamma phosphate does not simply float away, but is actually attached to the pump protein. As with most cases of ATP hydrolysis, a phosphate from ATP transfers onto another molecule. In a phosphorylated state, the Na + /K + pump has more free energy and is triggered to undergo a conformational change. This change allows it to release Na + to the cell’s outside. It then binds extracellular K + , which, through another conformational change, causes the phosphate group to detach from the pump. This detachment of the phosphate group triggers the K + to release to the cell’s inside. Essentially, the energy released from the ATP hydrolysis provides the energy required to power the pump and transport Na + and K + ions. ATP performs cellular work using this basic form of energy coupling through phosphorylation.
the energy currency molecule of cells; provides energy for most biological reactions that require an input of energy